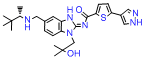
Quality Management
1. QA Management
Quality Assurance (QA) and Quality Control (QC) Operations have been in place for several years to insure that products at Excenen Pharmatech are prepared responsibly and in accordance with ISO 9001 standards. With QA available in every shift, compliance to the demands of cGMP is now in-use. Our pharmaceutical/biotech customers are welcomed and considered an integral part of our commitment to continuous quality improvement. QA system functions:- Quality of the in-coming materials
- In-process quality control
- Batch analysis
- Ware-house labeling
- Release control
- Batch sample storage
2. QC Management
Analytical support for lab and production ranges from the release of raw materials to in-process control. This includes the development of chromatographic methods as well as structural determination of impurities and by-products for release testing. Validation of analytical methods, and the preparation and characterization of reference standards are also available for more advanced projects. Major analytical equipment on-site:- HPLC: Agliant
- GC: Agilant
- LC/MS: Waters
- GC/MS: HP
- 400Hz NMR: Contract






 Biochemicals
Biochemicals