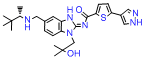
Product Information:
| Cat. No.: EX-A7750 | Purity: >98% |
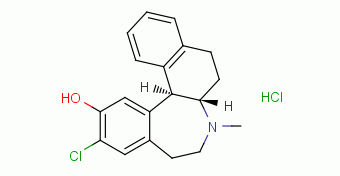 | |
| Chemical structure of Sch 39166 hydrochloride |
| Size | Unit Price (US$) | Stock | Quantity | Order |
|---|
| Send PO to E-mail:order@excenen.com; or Fax:+86 020 81619670 |
| Discount Request |
| Synonyms | Ecopipam hydrochloride |
|---|---|
| Synonyms 2 | Ecopipam HCl |
| CAS No. | 190133-94-9 |
| Purity | >98% |
| Formula | C19H21Cl2NO |
| Mol Weight | 350.282 |
| Appearance | solid powder |
| Shelf Life | >2 years if stored properly |
| Storage | Powder -20℃ 2 years; In solvent -20℃ 1 month; |
| Shipping | Shipped under ambient temperature as non-hazardous chemical. This product is stable enough for a few weeks during ordinary shipping and time spent in Customs. |
| Reference: | |
| CoA | CoA of Sch 39166 hydrochloride |
| Smiles: | OC1=C(Cl)C=C2C([C@]3([H])[C@@](CCC4=C3C=CC=C4)([H])N(C)CC2)=C1.[H]Cl |
|---|
KEYWORDS: buy Sch 39166 hydrochloride | Sch 39166 hydrochloride supplier | purchase | cost | manufacturer | order | distributor | buy 190133-94-9 | 190133-94-9 supplier






 Inhibitor Specialist
Inhibitor Specialist